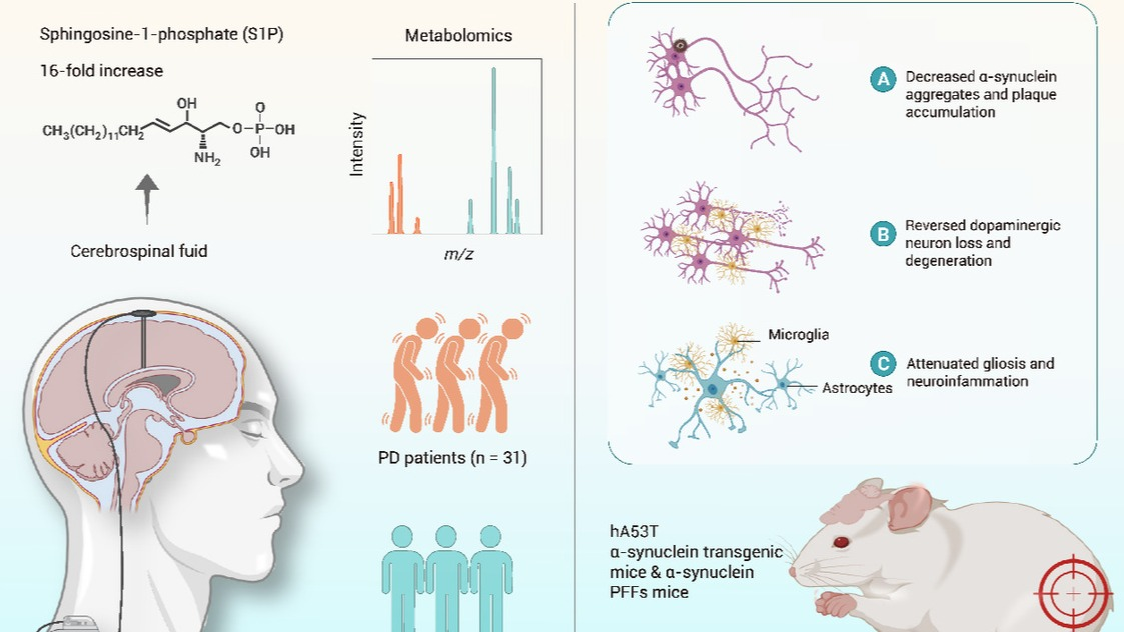
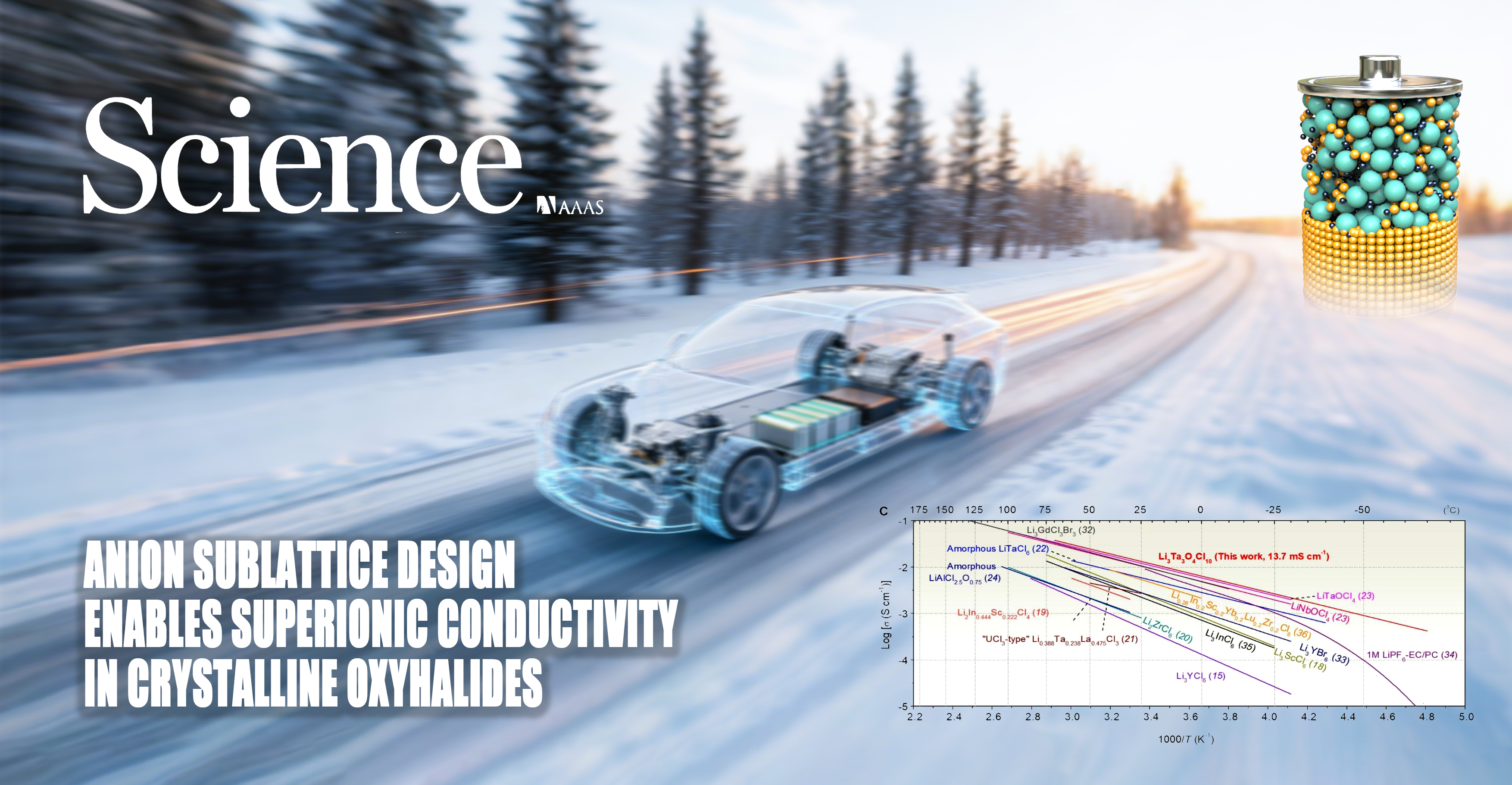
In the critical phase towards the practical application of all-solid-state battery technology, organic cathode materials have garnered widespread attention due to their environmental sustainability and tunable molecular structures. However, their practical application has been limited by bottlenecks such as low operating voltage and high solubility. Professor Xueliang Sun, a Chair Professor at Eastern Institute of Technology, Ningbo, in collaboration with other researchers, has proposed a novel cathode design strategy centered on regulation of the solid solvation structure. This research achievement has been published in Nature Chemistry. 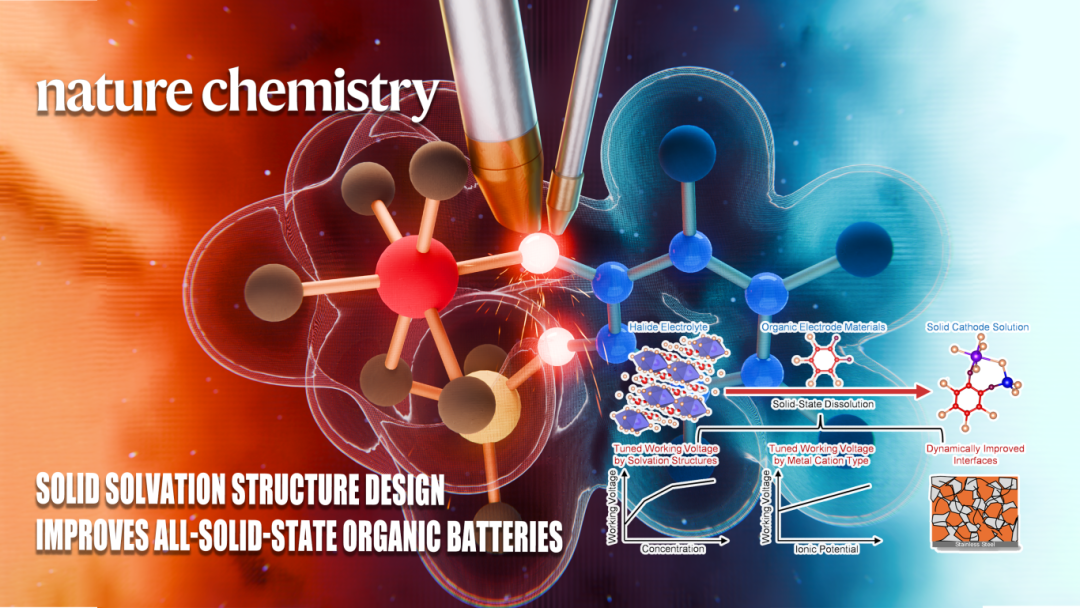
On August 4th, the team led by Professor Xueliang Sun published their work titled "Solid solvation structure design improves all-solid-state organic batteries" in Nature Chemistry. This study reports the use of an n-type organic small molecule cathode material as a solid solvent, introducing dual ions to construct a heteronuclear solid solvation structure. This approach activates a ligand-metal-ligand-charge transfer channel, achieving a high operating voltage of 3.6 V, an ultra-long cycle life exceeding 7500 cycles, and excellent rate capability. This work introduces the concept of the solid solvation structure, establishing a synergistic regulation theory connecting molecular structure, electronic structure, and electrochemical performance. It offers a new pathway for all-solid-state batteries characterized by sustainability, high performance, and low cost.

All-solid-state batteries are hailed as the "crown" of next-generation energy storage technologies, demonstrating potential far exceeding that of current liquid lithium-ion batteries in terms of energy density, safety, and cycle life. Nonetheless, realizing this vision still faces significant challenges within the cathode system of all-solid-state batteries. Particularly in the context of seeking green and sustainable material systems, organic cathodes have become a research hotspot due to their advantages, including tunable molecular structure, resource abundance, and ease of recycling. However, organic materials also confront two major bottlenecks:
Low Operating Voltage: The operating voltages of mainstream n-type organic cathode materials are generally below 3 V, making it difficult to meet the requirements for high energy density.
Capacity Fade: Small molecule organic materials are highly susceptible to dissolution in liquid electrolytes, leading to electrode structural collapse and rapid capacity decay. Although previous studies have attempted to mitigate these issues through molecular modification, polymerization strategies, or constructing organic salts, it remains challenging to simultaneously enhance voltage while maintaining cycle life and structural stability. Therefore, achieving organic cathodes with high voltage, long cycle life, and stable operation remains a key hurdle limiting their application in all-solid-state batteries.
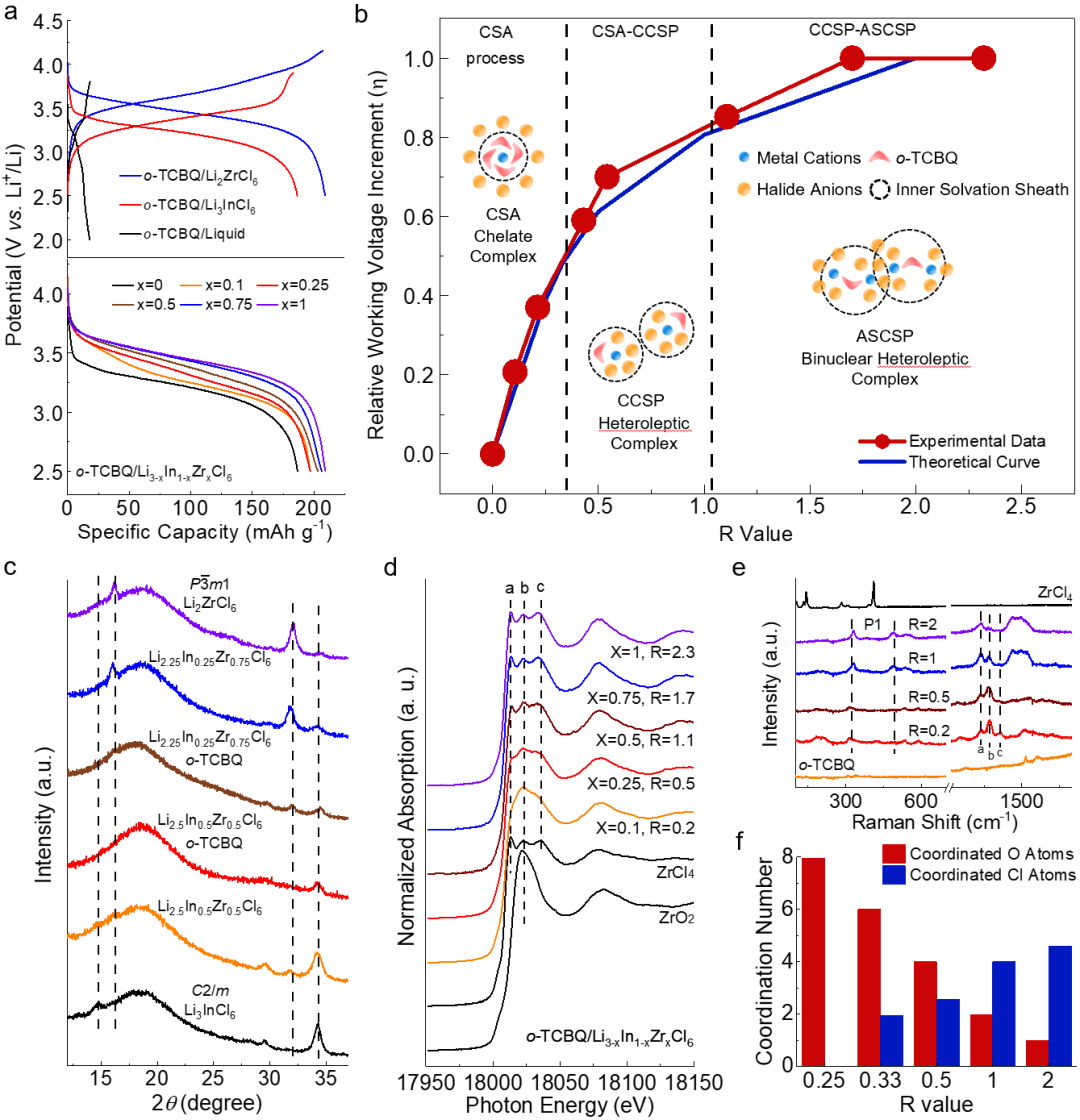 Figure 1 | Relationship between operating voltage and the evolution of the solid solvation structure
Figure 1 | Relationship between operating voltage and the evolution of the solid solvation structure
In recent years, Professor Xueliang Sun's team has dedicated extensive research to the application of halide solid-state electrolytes in all-solid-state batteries, developing a series of halide solid-state electrolyte materials with high ionic conductivity and high stability (published in Nature, 2025; Nat. Mater., 2025; Angew. Chem. Int. Ed., 2025; Adv. Mater., 2024; Nat. Commun., 2023; J. Am. Chem. Soc., 2023). Building on the understanding of ion transport mechanisms, they have established a structure-activity relationship for halides with a certain degree of universality (Nat. Commun., 2023; Nat. Commun., 2024).
Leveraging this strong foundation in halide solid-state electrolyte research, Professor Xueliang Sun's team proposed an innovative strategy centered on regulation of the solid solvation structure. In this study, the team innovatively utilized the organic small molecule o-TCBQ as a solid solvent, allowing it to form coordination structures with metal ions from the halide solid-state electrolyte. By modulating the organic-inorganic interactions, they constructed a tunable solvation structure, enabling deep regulation of the electronic structure and redox behavior of the organic molecule (Fig. 1).
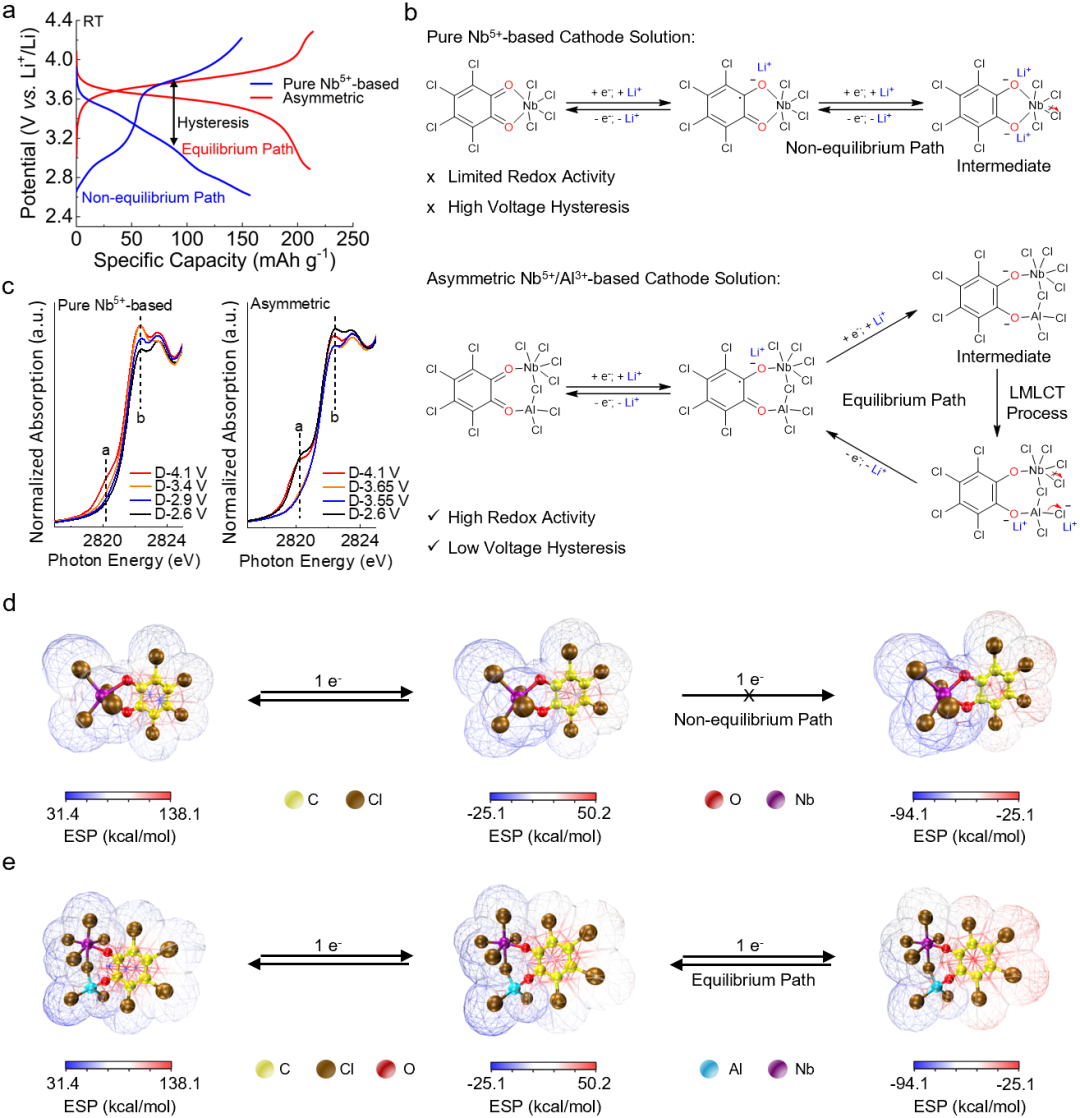
The study found that as ions like Zr⁴⁺ gradually entered the organic phase, forming multi-center coordination with the C=O groups of o-TCBQ, it not only induced electron delocalization towards the inorganic Cl ligands but also significantly enhanced the molecular voltage and redox activity. Further introduction of Nb⁵⁺ and Al³⁺ to construct a heteronuclear, heteroleptic ASCSP structure activated a ligand-metal-ligand charge transfer mechanism. This breakthrough overcame the limitations of interrupted reduction and voltage hysteresis typical of conventional organic electrodes, achieving a high operating voltage of 3.6 V in the all-solid-state system, a significantly reduced voltage hysteresis (700 → 180 mV), and enhanced reversibility of the two-electron reaction (Fig. 2). Benefiting from the optimized solvation structure and the novel electron transfer mechanism, this solid cathode solution system not only possesses high voltage and fast kinetics but also exhibits exceptional long-cycle performance and rate capability even under low stack pressure. Importantly, owing to the low Young's modulus of o-TCBQ and the electrostatic interactions with the halide electrolyte, the material can adaptively embed into the electrolyte grain boundaries during charge/discharge processes, achieving crack repair and enhanced electrical contact, demonstrating self-healing interfacial characteristics.
This work validates that solid solvation structure design is a highly promising technical pathway for realizing n-type organic cathode materials with high voltage and long cycle life in all-solid-state batteries. The research team believes that this strategy not only breaks through the core bottlenecks of organic electrodes—low voltage, short lifespan, and weak interfaces—but more importantly, it achieves systematic optimization of electronic structure and interfacial behavior through molecular-level structural regulation, laying a theoretical and technical foundation for the large-scale application of organic electrode materials. This work provides a novel solution for constructing all-solid-state battery systems with excellent cycling stability and sustainability, promising broad application in future electric vehicles, wearable devices, and large-scale energy storage. The research team will further explore the synergistic mechanisms of various organic molecules combined with heteronuclear metals and promote the scalable preparation and industrial compatibility of the material system, providing sustained momentum for the development of next-generation green solid-state battery technologies.
Yang Hu (PhD candidate, Western University, Canada), Dr. Han Su (Zhejiang University), and Dr. Jiamin Fu (Postdoctoral Fellow, Western University, Canada) are co-first authors of the paper. Assistant Professor Changhong Wang and Chair Professor Xueliang Sun from Eastern Institute of Technology, Ningbo are co-corresponding authors. The work received characterization support from Dr. Nian Zhang at the Shanghai Synchrotron Radiation Facility (SSRF) and from Ning Chen and Mohsen Shakouri at the Canadian Light Source Inc.