In liquid electrolytes, salts are "dissolved" by solvents, allowing ions to move freely. In solids, however, everything appears static and “dissolution” should not occur. Yet, this research precisely makes solids "learn" to "dissolve".
A research team from Eastern Institute of Technology, Ningbo has discovered a class of halide van der Waals materials. These materials act like "solid solvents," disassembling metal salts into freely mobile ions within the solid structure—a phenomenon they term "Solid Dissociation".

A team led by Chair Professor Xueliang Sun, Foreign Member of the Chinese Academy of Engineering, and Associate Professor Xiaona Li at the Eastern Institute of Technology, Ningbo, along with collaborators, has proposed and validated a novel design concept for solid-state electrolytes—Solid Dissociation. This strategy utilizes halide van der Waals materials as solid solvents to dissolve various metal salts through strong coordination interactions, directly generating amorphous, homogeneous solid materials with high ionic conductivity. This approach constructs an amorphous solid electrolyte system characterized by high ionic conductivity and broad compositional tunability, providing high-performance, customizable solutions for all-solid-state batteries under various operating conditions, potentially accelerating their commercialization.
The related research findings were published in Nature Energy on October 20th (Beijing Time). The Eastern Institute of Technology, Ningbo is the first completing institution.
In traditional liquid electrolyte-based lithium and sodium-ion batteries, most solid-state electrolytes are crystalline ionic conductors whose ion transport properties heavily depend on their lattice structure, preventing free compositional design akin to liquid systems. How to break away from lattice dependency and broaden the design freedom for material composition has become a significant challenge in solid-state electrolyte research.
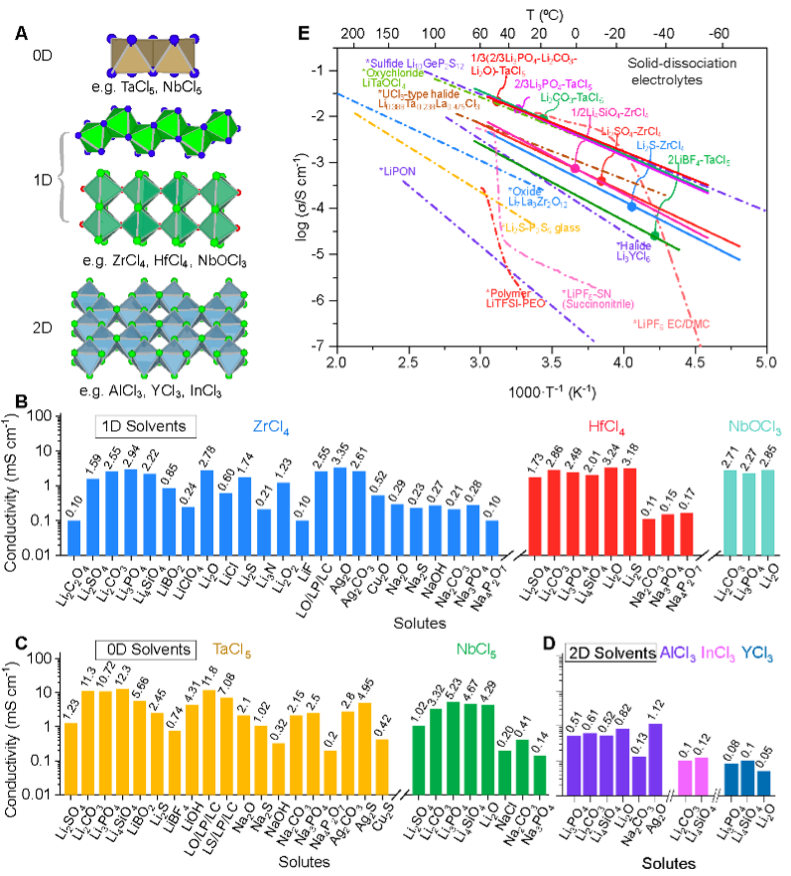
Building upon the long-term research of Professor Xueliang Sun and Professor Xiaona Li teams in the field of halide ionic superconductors, the team proposed this new concept of Solid Dissociation for constructing solid electrolytes. This strategy employs halide van der Waals materials (MClx, where M includes Ta, Nb, Zr, Hf, Al, Y, In, etc.) as solid solvents. These crystals dissolve various metal salts through strong coordination interactions, directly yielding amorphous, homogeneous solid materials with high ionic conductivity.
Schematic of the Solid Dissociation Electrolyte System | Image provided by the research team.
Using this method, the team has successfully prepared over 70 novel solid-state electrolytes capable of conducting different metal ions (e.g., Li+, Na+, Ag+). Among these, more than 40 exhibit room-temperature ionic conductivities in the range of 10⁻³ to 10⁻² S/cm. This significantly expands the compositional design space for solid-state electrolytes and also provides a new platform for exploring ion transport mechanisms.
Research indicates that halide van der Waals materials are composed of low-dimensional [MCl₆]ₙ octahedral units stacked via van der Waals forces. The strong interaction between the Lewis acidic metal cations in these crystals and the anions of the salts (e.g., SO₄²⁻) triggers a liquid-phase-like dissolution-dissociation process. The propensity of these low-dimensional units to migrate and rearrange under mechanochemical action facilitates ion diffusion during the solid-state synthesis process.
Leveraging this mechanism, the research team introduced functional elements like nitrogen and fluorine without sacrificing ion transport performance, enabling material customization for various application scenarios. For instance, they developed electrolytes suitable for 15C rate charging/discharging and operation at low temperatures down to −50°C. Materials with high fluorine content can be stably matched with high-voltage cathodes (up to 4.8 V). Introducing anions such as phosphate and sulfate significantly enhanced air stability and compatibility with dry room processing. Concurrently, materials based on Zr, Al, and Nb offer cost advantages over conventional solid electrolytes, bringing them closer to the requirements for large-scale applications.
This achievement not only brings unprecedented compositional tunability to all-solid-state electrolytes but also establishes a rich candidate material library, laying a solid foundation for the development of high-performance all-solid-state batteries. The research team stated that future work will focus on expanding the range of dissolvable salts, particularly lithium salts with larger anions (e.g., LiFSI, LiTFSI), and delving deeper into the relationship between medium-range connectivity within the local structure and electrochemical performance. Furthermore, this Solid Dissociation strategy could be extended to fields such as amorphous cathode materials and even chemical sensors.
Junyi Yue, a PhD candidate in the Hong Kong Polytechnic University- Eastern Institute of Technology, Ningbo joint program, and Simeng Zhang, an Associate Researcher at the Ningbo Institute of Digital Twin, are co-first authors of the paper. Xueliang Sun, Xiaona Li, and Jianwen Liang, Director of the Solid State Batteries Research Center at GRINM Guangdong Institute for Advanced Materials and Technology, are corresponding authors. The research received beamtime support for synchrotron radiation X-ray experiments from the Canadian Light Source and the Shanghai Synchrotron Radiation Facility. It also received support from GRINM Guangdong Institute, the Institute of Physics (Chinese Academy of Sciences), and collaborators at the Hong Kong Polytechnic University in material synthesis and characterization.